On November 2, 2023, the Centers for Medicare & Medicaid Services (CMS) finalized the Outpatient Prospective Payment System (OPPS) and the Ambulatory Surgical Center (ASC) Payment System for calendar year (CY) 2024. CMS moved forward with adopting the Risk-Standardized Patient Reported Outcome-Based Performance Measure (PRO-PM) Following Elective Primary Total Hip Arthroplasty (THA) and/or Total Knee Arthroplasty (TKA) in the ASC Setting (THA/TKA PRO-PM). The only change from the proposed ruling related to this measure was a modification to extend the voluntary reporting through CY 2027 followed by mandatory reporting beginning one year later than originally proposed with the CY 2028 reporting period.
According to CMS, the decision to extend the voluntary reporting period and postpone mandatory reporting by one year stems from feedback they received. Many commenters expressed support for the measure but recommended adjustments to the proposed timelines in order to allow more time for the initial implementation of PROs collection. CMS acknowledged the potential burden and initial implementation resources required for collecting PROMs data, especially compared to some other types of quality measures. However, they also stressed the importance of PROs for both clinical decision-making and shared decision-making with patients. CMS intends to closely monitor progress during implementation, particularly in terms of data collection burden, and will allow future rulemaking to address any necessary improvements.

With a growing number of THA and TKA procedures taking place in outpatient settings, the adoption of the PRO-PM as part of the Hospital Outpatient Quality Reporting (OQR) programs and the ASC Quality Reporting (ASCQR) programs demonstrates the need to ensure that high-quality PRO data accompanies this shift. See our previous blog for more background information on the payment systems and the reporting programs. The following is the finalized information on the PRO-PM:
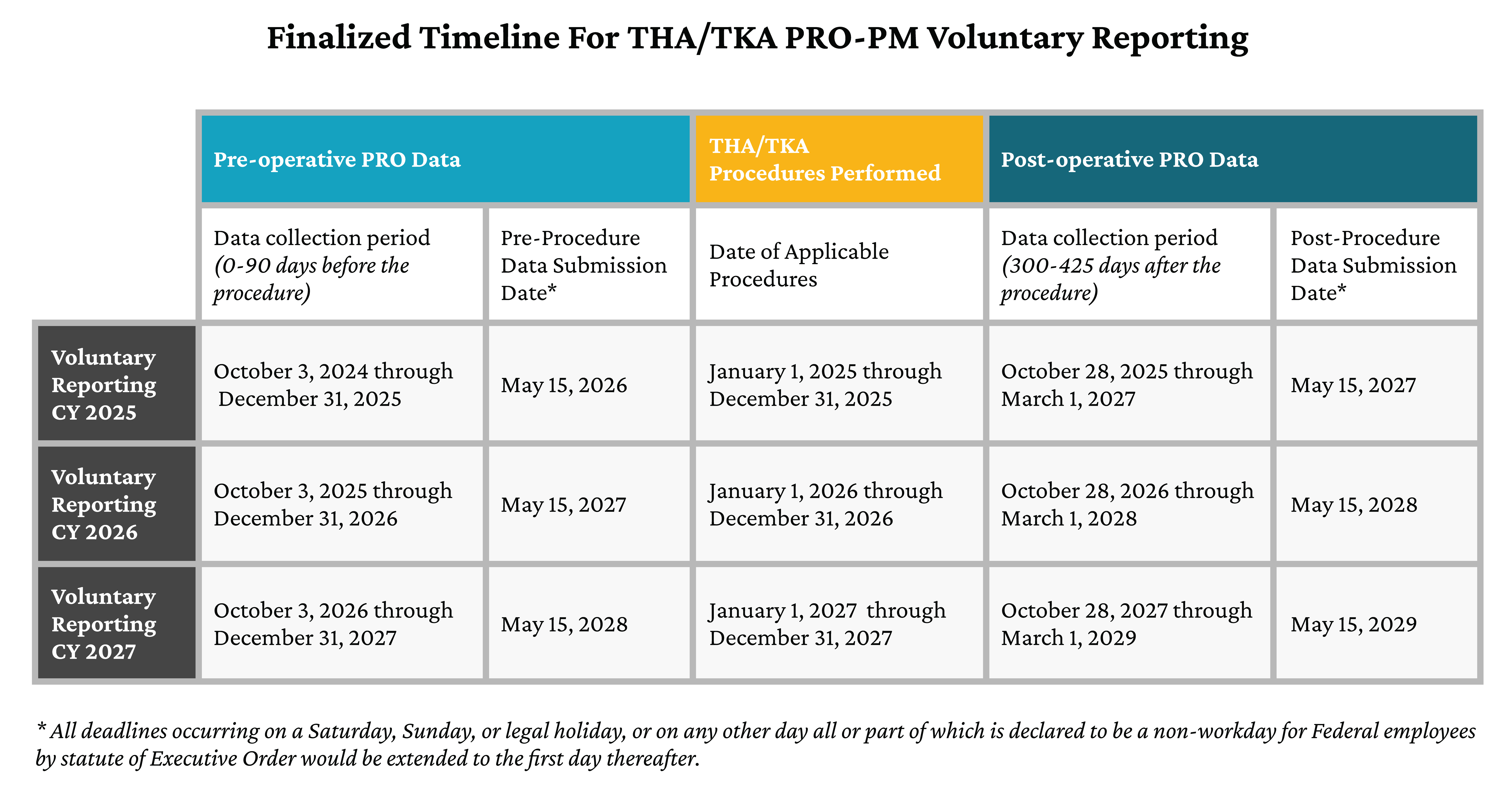
Finalized Reporting Periods:
For the first voluntary reporting period for CY 2025:
- The pre-operative PRO data collection window will be from October 3, 2024 through December 31, 2025 (90 to 0 days before the procedures) with a submission due date of May 15, 2026
- The performance period is for eligible elective THA/TKA procedures performed from January 1, 2025, through December 31, 2025
- The post-operative PRO data collection window will be from October 28, 2025 through March 1, 2027 (300 to 425 days after the procedure) with a submission due date of May 15, 2027.
For the second voluntary reporting period for CY 2026:
- The pre-operative PRO data collection window will be from October 3, 2025 through December 31, 2026 (90 to 0 days before the procedures) with a submission due date of May 15, 2027
- The performance period is for eligible elective THA/TKA procedures performed from January 1, 2026, through December 31, 2026
- The post-operative PRO data collection window will be from October 28, 2026 through February 29, 2028 (300 to 425 days after the procedure) with a submission due date of May 15, 2028
For the third voluntary reporting period for CY 2027:
- The pre-operative PRO data collection window will be from October 3, 2026 through December 31, 2027 (90 to 0 days before the procedures) with a submission due date of May 15, 2028
- The performance period is for eligible elective THA/TKA procedures performed from January 1, 2027, through December 31, 2027
- The post-operative PRO data collection window will be from October 28, 2027 through February 28, 2029 (300 to 425 days after the procedure) with a submission due date of May 15, 2029
For the mandatory reporting period for CY 2028:
- The pre-operative PRO data collection window will be from October 3, 2027 through December 31, 2028 (90 to 0 days before the procedures) with a submission due date of May 15, 2029
- The performance period is for eligible elective THA/TKA procedures performed from January 1, 2028, through December 31, 2028
- The post-operative PRO data collection window will be from October 28, 2028 through February 28, 2030 (300 to 425 days after the procedure) with a submission due date of May 15, 2030

Impacted HOPDs/ASCs
All HOPDs and ASCs that perform elective primary THA and TKAs will be impacted by the PRO-PM. However, the following are excluded:
- Critical access hospitals (CAHs)*
- Hospitals located in Maryland and paid under Maryland’s All-Payer or Total Cost of Care Model
- Hospitals located outside of the 50 States, the District of Columbia, and Puerto Rico
- Indian Health Service (IHS) hospitals
*Note that while CAHs are excluded, CMS strongly recommends that CAHs participate in the measure and voluntarily submit the data for quality improvement.
Patient Inclusion/Exclusion
The THA/TKA PRO-PM includes patients who:
- Underwent an elective primary outpatient THA/TKA procedure that is performed in an HOPD or ASC
- Are enrolled in Medicare FFS Part A and B for the 12 months prior to the date of the procedure and in Medicare Part A and B during the procedure
- Are aged 65 years or older
The measure excludes patients with:
- Fractures
- Revisions
- Discontinued procedures (procedures that were started but not completed)
Data Sources
The THA/TKA PRO-PM uses four (4) data sources for calculation of the measure:
- PRO data
- Claims data
- Medicare enrollment and beneficiary data
- U.S. Census Bureau survey data
PRO Data includes:
- PROMIS Global (mental health subscale items) or VR–12 (mental health subscale items)
- HOOS, JR (for THA patients) or KOOS, JR (for TKA patients)
- Single-Item Health Literacy Screening (SILS2) questionnaire
- Total painful joint count (patient reported in non-operative lower extremity joint)
- Quantified spinal pain (patient-reported back pain, Oswestry index question)
Collection Intervals:
- PRO data is collected both before and after THA/TKA at the following collection intervals:
- Pre-operatively (90 to 0 days before surgery); and
- Post-operatively (300 to 425 days after surgery)
Data Submission
HOPDs and ASCs will submit the data sources collected pre- and post-operatively for each eligible patient using the HQR System. The system allows for the data to be submitted using CSV, XML, or there is a manual data entry option. Similar to the IQR program, HOPDs and ASCs can either choose to submit their data to CMS directly or utilize a vendor or registry.
Success Criteria
During mandatory reporting in the Hospital OQR and ASCQR programs, HOPDs will be required to submit pre-op and post-op data for at least 50 percent of eligible procedures and ASCs will be required to submit pre-op and post-op data for at least 45 percent of eligible procedures. Hospitals and ASCs that do not participate or fail to meet these requirements may receive a two percent reduction of their annual payment update (APU).
The measure outcome is the RSIR proportion of patients who underwent elective primary THA/TKA and met or exceeded a substantial clinical improvement threshold between pre-op and post-op. Improvement is defined as:
- For THA patients- meeting or exceeding the threshold of 22 points on the HOOS, JR. between pre-op and post-op
- For TKA patients- meeting or exceeding the threshold of 20 points on the KOOS, JR. between pre-op and post-op
Simplify The Complexities Of The PRO-PM Under The OPPS Rule
Our expert team paves the most efficient route for PRO-PM reporting success: ensuring a seamless implementation in just 30 days and sparing you the burden of staff-intensive efforts.




